Even at ultracold temperatures close to absolute zero, molecules can undergo chemical reaction. These reactions follow the principles of quantum physics and manifest themselves in fascinating and yet unexplained phenomena.
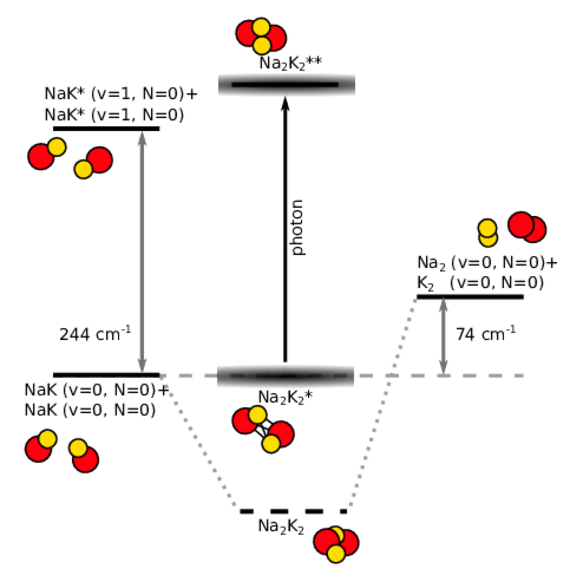
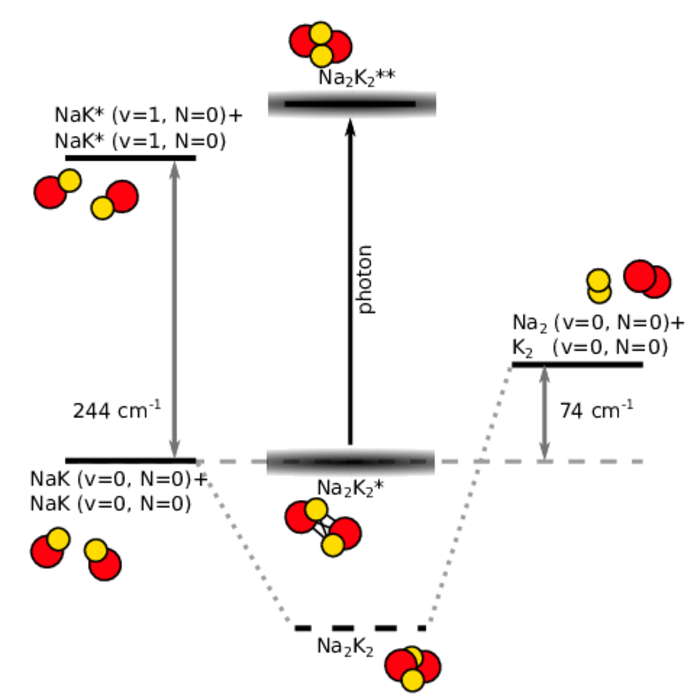
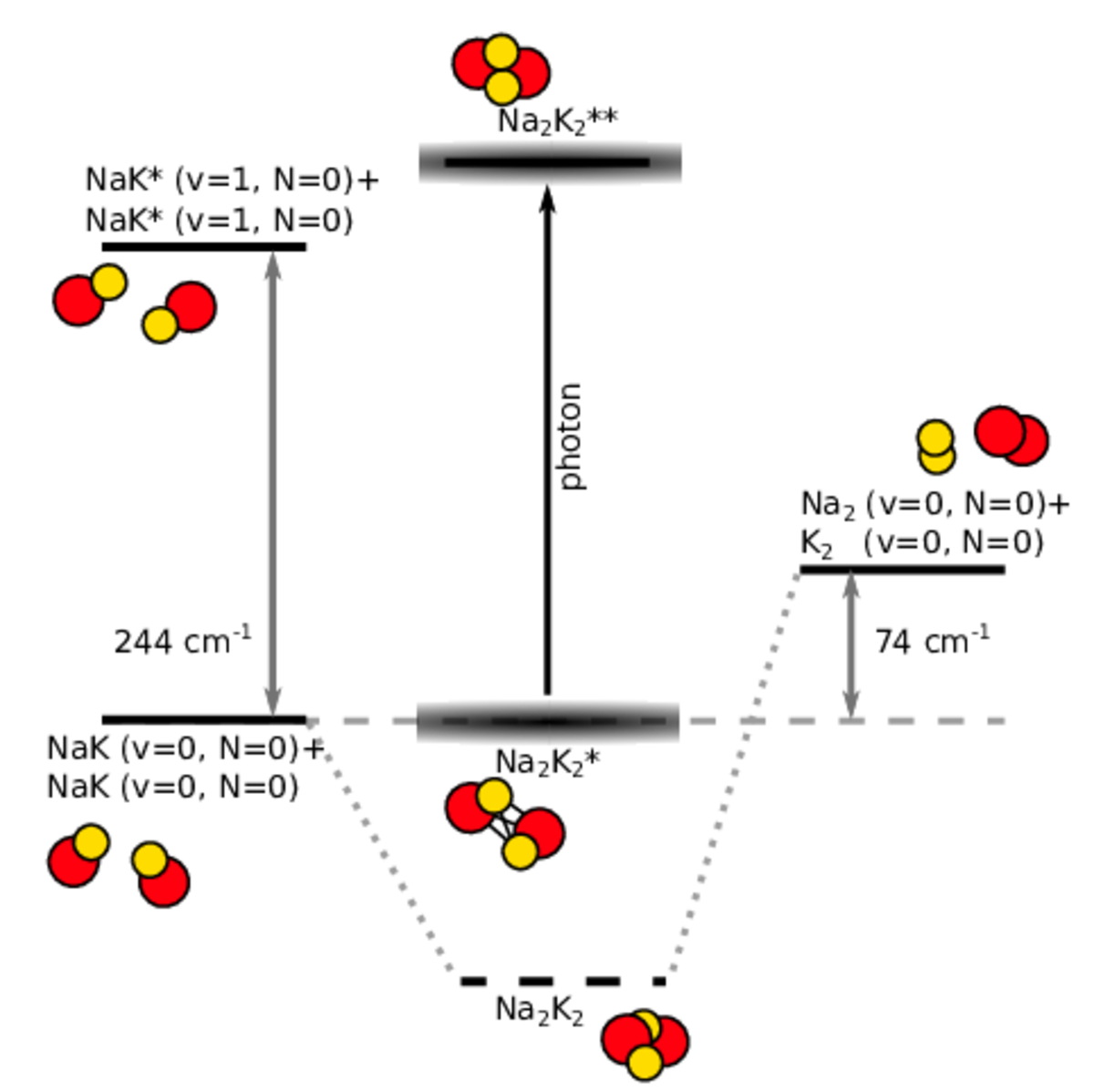
Ultracold diatomic ground-state molecules are ideal candidates for the search of quantum chemical phenomena. They possess a rich internal level structure which gives rise to many interesting effects. One intriguing effect is the formation of a long-lived complex, when two molecules collide. Due to resonant effects of the internal level structure, the two colliding molecules (e.g. NaK+NaK) stick together (Na2K2*) for a significantly long time before they detach again. During the enhanced collision time, the sticky complex might change its quantum state, for example by photoexcitation of light (Na2K2**) or spontaneous spin-relaxation (Na2K2).
Another way to investigate ultracold chemical reaction is to induce chemical exchange reactions in otherwise chemically stable molecular ensembles. Bialkali ground-state molecules, such as NaK, can be excited selectively to a higher vibrational state of the internal level structure. In a collision, those excited molecules NaK*+NaK* can undergo the exchange-reaction to the products Na2 + K2. This simple model reaction exhibits only a limited amount of reactant and product states which can be used to study and model the fundamental physics of chemical reaction on the basis of quantum mechanics.
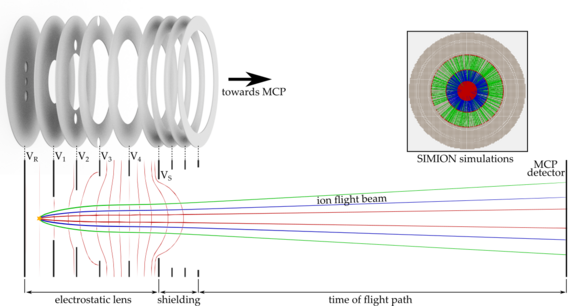
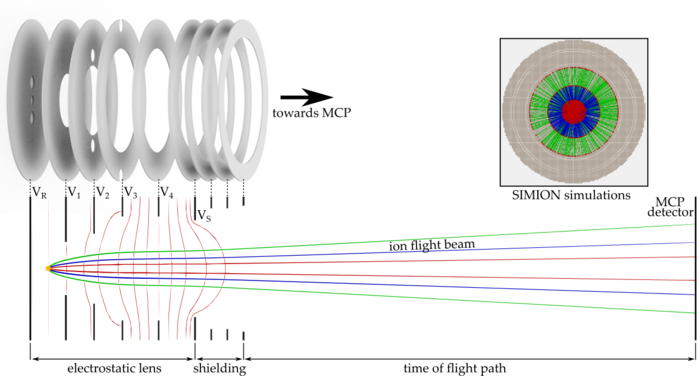
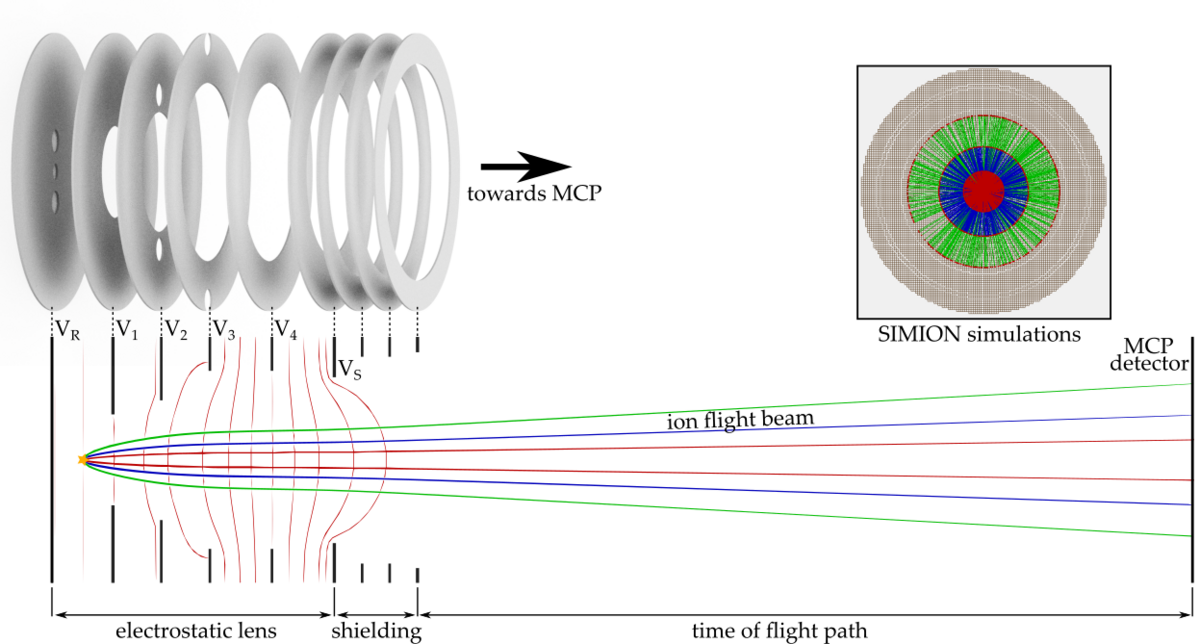
To investigate these effects, we are setting up a new apparatus based on an imaging technique known from cold chemistry experiments called “time of flight-velocity map imaging-mass spectrometry” (TOF-VMI-MS). We aim for the detection of all particles and their states involved in the collision of ultracold molecules. We use a short UV laser pulse to ionize and fragmentize the particles. The resulting fragments carry residual kinetic energy (velocity) which is dependent on the particles initial state. An electrostatic lens setup accelerates the ions towards an ion detector plate while mapping different velocity classes onto each other (velocity map imaging, VMI). The free expansion of the ion cloud during its time of flight (TOF) towards the detector helps to separate the different velocity classes. Due to acceleration of ions with different masses, the ion cloud also separates according to their charge-to-mass ratio, which allows us to use this detection scheme as a mass spectrometer.
The Team
Principle Investigators


Institut für Quantenoptik
30167 Hannover


Institut für Quantenoptik
30167 Hannover
Ph.D. Students
Institut für Quantenoptik
30167 Hannover


